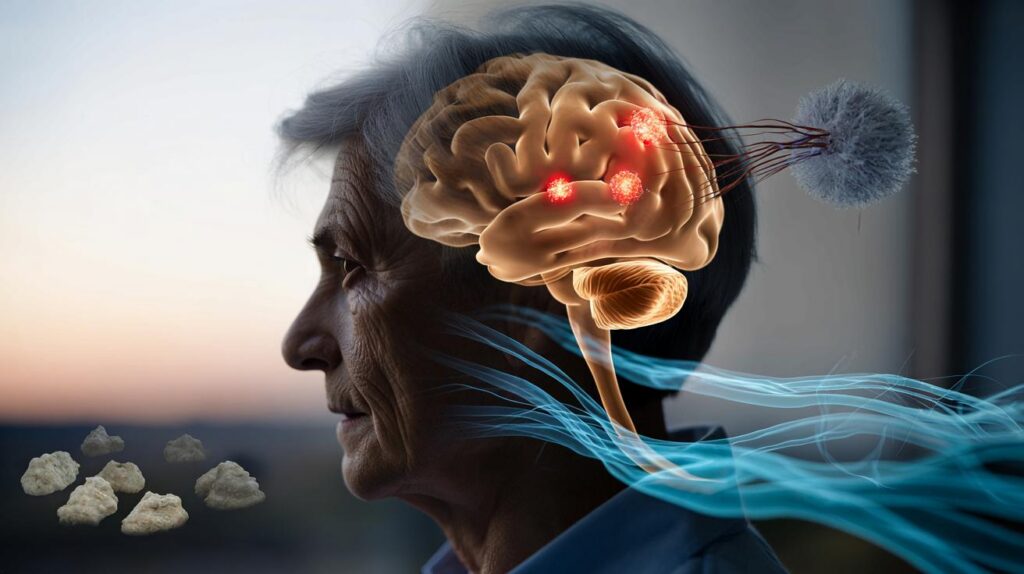
For years, the story of Alzheimer’s has sounded simple and bleak: sticky plaques build up, brain cells die, memories fade. Drugs chased those plaques, families waited, and progress limped along. Then a consortium of labs peered closer at what happens just before the damage, in the living circuitry where thoughts spark. What they saw was not sludge but a signal. An immune miscue. A clean‑up tag slapped on healthy synapses, as if the brain were spring‑cleaning the very connections that make us who we are. That changes everything—if it holds.
I was standing at the back of a memory clinic when a man started humming. His wife was rummaging for the appointment letter; his eyes were on the carpet; the tune—ELO, a summer from the seventies—drifted in, as bright as a postcard. We’ve all had that moment where a song hooks a memory you thought was gone. The nurse smiled and asked what it brought back. He said, “The smell of my dad’s car.” Not the registration plate. Not his childhood address. A smell, precise and warm. What if Alzheimer’s isn’t forgetting at all?
From sticky plaques to a misfiring clean‑up crew
The idea jolting labs this year is disarmingly simple: the brain’s immune system may be tagging working synapses as trash. Under stress—poor sleep, infection, vascular strain—soluble fragments of amyloid appear to act less like gunk and more like a siren. Microglia, the brain’s resident scavengers, hear it and start pruning. The signal spreads, and networks thin not because they’re clogged but because they’re being tidied away by mistake. The shock? The plaques aren’t the main problem. They might be gravestones, not the cause of death. That flips the field from demolition to de-escalation.
Look at the timing. In mice engineered to model early disease, memory falters before plaques loom on scans. In people, subtle attention slips can predate a clinical diagnosis by years, even with modest amyloid levels. One proteomic study flagged an uptick of complement proteins—the molecular “eat me” tags—long before wholesale neuron loss. There’s a pattern here: synapses are being marked and nibbled away when the outside looks almost normal. Families notice it in tiny turns: the recipe that goes awry, the left turn that becomes a loop. Hardly anyone sees a plaque. Everyone sees a pattern falling quiet.
Follow the logic and a new treatment target emerges. If soluble amyloid is the siren and microglia are the responders, the job is not scrubbing every last deposit but muting the false alarm and teaching the crew to stand down. Block the tag. Calm the pruning. Nudge the brain’s waste‑clearance rhythms back to night‑shift mode, when sleep pumps cerebrospinal fluid through perivascular channels like a gentle tide. Trials that only cleared plaques slowed decline a little, then hit safety snags. Different idea: keep the synapses, even if some residue remains. This changes the playbook.
What this could mean for treatment—starting now
The immediate move is deceptively modest: shift attention to the window before heavy damage, and measure the signals that govern pruning. Clinics are beginning to pair blood biomarkers—tau fragments, inflammatory signatures—with sleep and vascular assessments, building a picture of who’s in the “signal zone.” That enables targeted trials of agents that tamp down complement activity, boost glymphatic flow at night, or stabilise microglial states. Think less sledgehammer, more dimmer switch. *For once, the story isn’t about what we can’t fix.* It’s about catching a faulty instruction and rewriting it while the network is still there.
There’s a very human rhythm to this. People don’t live in labs; they live in routines. Small choices that cool the immune system—consistent sleep, daylight walking, gum health, blood pressure under control—act like sandbags along a rising river. Let’s be honest: nobody does this every day. You’re juggling families, jobs, appointments, fear. The point is not perfection. It’s creating more nights when the brain can rinse and reset, fewer days when microglia hear a false siren. That’s not a miracle cure. It’s a baseline that gives tomorrow’s drugs a fighting chance.
Scientists are trying to find a quiet, sustainable way out of the noise we’ve made around Alzheimer’s. The window for help might be wider than we feared. They talk less about silver bullets, more about coordinated nudges—immune, vascular, behavioural—stacked early. You can hear the relief in some voices.
“We’ve been chasing the tombstones,” one neuroscientist told me, “when the murder weapon was the wrong instruction all along.”
- Ask your GP about emerging blood tests that track neuroinflammation alongside amyloid and tau.
- Prioritise sleep regularity and daylight exposure for circadian alignment; it’s a low‑friction lever on glymphatic clearance.
- Treat gum disease promptly; oral pathogens can amplify brain immune signalling via the bloodstream.
- If you’re eligible, look into trials targeting complement or microglial pathways, not just plaque load.
The bigger picture
Every big shift in medicine starts with a story rewrite. Heart disease moved from fate to plumbing; HIV from fatal to chronic; cancer from monolith to mosaic. Alzheimer’s is now stepping into its own reframing, and it lands closer to everyday life than you’d think. A misfiring clean‑up crew can be quieted. A tide that stalls can be coaxed to move. A lab signal can become a clinic tool, and a clinic tool can become something families feel in the kitchen at 8pm, when the recipe stays on track and the conversation doesn’t fog. This isn’t hype. It’s a wager on timing, and on the honesty to change course while the map is still smudgy. The science is still writing. So are we.
| Key points | Details | Interest for reader |
|---|---|---|
| Immune mis‑tagging of synapses | Soluble amyloid and complement proteins may mark healthy synapses for removal, prompting microglial pruning | Explains early symptoms and points to targets beyond plaque removal |
| Shift to early, layered interventions | Blood biomarkers, sleep and vascular profiling guide trials that calm immune noise and support clearance | Offers practical steps today while new therapies are tested |
| Plausible path to gentler drugs | Focus on dimming false alarms rather than scraping the brain clean of deposits | Could mean safer treatments with meaningful daily impacts |
FAQ :
- What exactly did scientists find?They observed signals—especially complement “eat me” tags—appearing on working synapses alongside soluble amyloid, which drove microglia to prune connections that should have been kept. The discovery reframes early Alzheimer’s as a mistaken clean‑up job rather than just a build‑up of plaques.
- Does this mean a cure is close?Not yet. It offers a clearer target and a better moment to act. Therapies that modulate these immune signals and support the brain’s night‑time clearance are entering or expanding trials, with the hope of slowing decline more reliably.
- Should current plaque‑clearing drugs be stopped?No blanket advice fits everyone. Some people benefit from existing drugs. The new insight suggests combining or sequencing treatments—plaque lowering with immune‑calming approaches—might yield better outcomes over time. Speak with your clinician about your specific case.
- What can I do now that’s realistic?Stack small habits that reduce inflammatory “noise”: consistent sleep and light exposure, blood pressure control, oral health, gentle daily activity. These are not cures. They create conditions where the brain’s own clearing systems work as designed.
- How soon could this change standard care?If ongoing studies validate the markers and show benefit from targeting complement and microglial states, guidelines could begin to shift within a few years. Screening might move earlier, with layered care plans tailored to a person’s immune and sleep profile.


So are we just swapping the plaque story for a shiny new buzzword? What definitve human evidence shows complement tagging in vivo, beyond mouse models and proxies? Cautiously interested.